
Serum biochemistry revealed normal electrolyte concentrations (K, Ca, Mg, Na) and no acute kidney injury. There was no leukocytosis, fever or tachypnea to suggest sepsis. Electrocardiography and cardiac biomarkers again excluded myocardial ischemia. A total of three liters intravenous crystalloid fluid boluses were administered but there was only a transient rise in blood pressure (Fig. The patient was fully alert and oriented and expressed his wish to not have placement of any central venous access. The following morning, 8 h after administration, the patient was hypotensive with a blood pressure of 80/45 mmHg, pulse of 51 beats/min and had a respiratory rate of 18 breaths/min. The patient denied any chest pain and the overnight team excluded myocardial ischemia with cardiac biomarkers and electrocardiography. Throughout the night the patient remained asymptomatic but the heart rate had started to decline.

At 2 hours after administration, the blood pressure declined from 120/78 mmHg to 97/61 mmHg but the heart rate was unchanged. From this point on, the heart rate and blood pressure are charted in Fig. The first dose of carvedilol was administered after lunch and no reaction was noted acutely. The other medications being administered were rifaximin and pantoprazole, and a final dose of ceftriaxone (1 g/day for 7 days) given only as prophylaxis. Echocardiography on admission showed cardiac function was normal with a preserved ejection fraction.
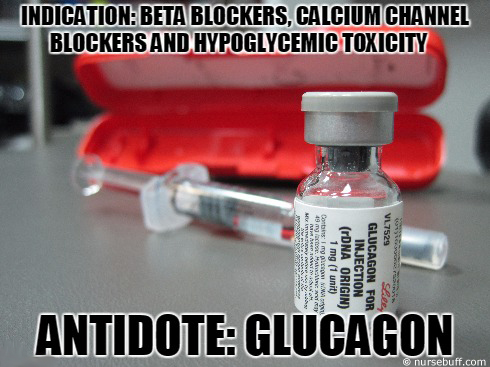
Post intervention, the patient had an uneventful course and 7 days after presentation carvedilol 12.5 mg twice daily orally was started. An esophagogastroduodenoscopy confirmed bleeding esophageal varices, and band ligation was performed. On imaging there was slight ascites and he did not have encephalopathy on examination, with Child-Pugh score of 7. Laboratory investigation and imaging confirmed cirrhosis, with an alanine aminotransferase (ALT) of 101 IU/L, aspartate aminotransferase (AST) of 51 IU/L, albumin of 2.9 g/dL, total bilirubin of 1.4 mg/dL and international normalized ratio (INR) of 1.4. He did not regularly see a primary care physician and did not take any medications prior to admission. The patient did not have diabetes, ischemic heart disease or other comorbidities. Finally, dose adjustment and slow uptitration of carvedilol in cirrhosis is recommended.Ī 56-year-old man with liver cirrhosis (secondary to hepatitis C) presented with hematemesis. Hospitals where carvedilol is used in patients with cirrhosis should have glucagon in formulary at doses to treat toxicity (bolus and infusion). Nurses and physicians need to recognize the toxidrome early. The typical threshold for carvedilol toxicity in overdose is 50 mg but in patients with cirrhosis this is not applicable.

Patients with cirrhosis represent a special at-risk group for beta blocker toxicity. The patient was successfully treated with glucagon 5 mg bolus followed by infusion. As there was no overdose, the diagnosis was based on clinical recognition of the toxidrome. We present a case of cardiogenic shock that occurred after carvedilol 25 mg orally was administered to a patient with cirrhosis. However, there are no dosage adjustments in the manufacturer’s labelling for mild to moderate hepatic impairment. Due to its extensive hepatic metabolism, carvedilol is contraindicated in severe hepatic impairment. The main cause of portal hypertension is cirrhosis and therefore carvedilol is increasingly used in these patients. It has been associated with improved outcomes regarding variceal bleeding, hepatic decompensation and death when compared to propranolol and endoscopic band ligation. Carvedilol is used in the management of hypertension, ischemic heart disease, heart failure and most recently, portal hypertension.


 0 kommentar(er)
0 kommentar(er)
